preventing issues linked to water quality
Reading time:Treatment strategy = implementation of tools. Using the right tools equates to limiting the environmental impact for optimum efficiency
Depending on the origin of the water (raw water), watercourse, lake, boreholes, wells, water table, drinking water (tap water) and its use on an industrial site, we will be required to perform a pre-treatment to give this raw water the necessary qualities for its future use and in order to not disrupt the good functioning of cooling towers. The “adapted” water introduced into the cooling towers will therefore be known as make-up water.
The pre-treatments which may be applied are:
- screening;
- coagulation, clarification;
- chlorination, de-chlorination (activated carbon);
- filtration;
- softening, de-alkalisation, reverse osmosis, de-mineralisation.
There are two main ways of considering water in a cooling tower, and more particularly air-cooling towers:
- process known as “natural balance” or with a “regulated pH”: This process consists in adjusting the pH and total alkali strength of the water in circulation, to ensure its calco-carbonic balance: Ryznar Index in the region of 6.6. This is obtained by the injection of strong acids (chlorohydric acid, sulphuric acid), and via the limitation of the concentration rate. This process is appealing due to its simplicity, but it has significant limitations, in particular:
- the diversity of temperatures within the circuit means that the water cannot be balanced at every point;
- a significant blowdown rate must be maintained to limit the concentration in dissolved salts in the water flow;
- the loss in CO2 from the tower destabilises the balance of the water.
- process known as “free pH”: pH is not regulated, its value increases in relation to make-up water. Water is left to balance itself according to cooling tower operating conditions. This balancing will result in scaling phenomena which must be prevented using chemical inhibitors.
The following sub-chapters describe the processes and the active substances which have a certain effect but which can reveal themselves to be insufficient or difficult to implement. It is for this reason that via the Aqualead® range, SUEZ proposes conditioning solutions based on particular formulations.
Perfecting a formulation must take account of:
- the substance or substances for the effect or effects expected;
- the necessary additives:
- for a better solubilisation;
- for a better storage stability or in the water to be treated;
- to reach the target more easily (surfactant);
- the possible dangerous nature of components and their possible environmental impacts;
- the cost of use including implementation using simple or elaborate equipment.
preventing scaling
Scaling can be prevented by:
- the modification of cooling tower operating conditions: increased flow rate, modification of materials, reduction in thermal exchanges …;
- managing elements causing scaling: total or partial softening, de-carbonation using resins or lime, acidification ;
- limiting concentration in the circuit, which reduces the salinity of water and the concentration in ions intervening in scaling phenomena.
However, these preventive measures are rarely sufficient to prevent scaling phenomena in cooling circuits. Conditioning is complementary and will provide an additional action.
inhibiting scaling
Some products possess anti-scaling properties as they act by disrupting scaling phenomena in various ways, mostly:
- sequestration or complexation: This reaction is stoichiometric, the divalent cations (Ca2+, Mg2+, Fe2+, Cu2+…) are complexed and can no longer participate in precipitation reactions with certain anions (HCO-3, CO2-3, SO2-4);
- threshold effect: it is an inhibition effect of the precipitation from very low “sub-stoichiometric” concentrations;
- modification of crystallisation: these inhibitors modify the form of the formed crystals which are then less adherent on surfaces;
- dispersion: by this action, the elements forming the scale specifically react electrochemically with a polymer. This keeps them dispersed and avoids their agglomeration and precipitations.
main scaling inhibitors
- Chelating agents
A chelating agent acts by complexation or sequestration.
- Polyphosphates
Polyphosphates absorb on to crystallisation seeds and are considered to be crystal modifiers.
Their stability varies according to temperature, when T > 60°C, the molecules hydrolyse into orthophosphates, which do not have the same anti-scaling effect. Orthophosphates are easily metabolised by bacteria which can favour bacterial proliferation.
- Phosphonates
Phosphonates act via threshold effect.
Their stability in relation to temperature is good up to 180°C.
The main active substances do not all have the same sensitivity to oxidizing biocides which can reduce their effectiveness and impose an over-dosage of biocide.
- Polymers
This term regroups a wide diversity of products. They act by dispersion.
In particular, we can distinguish them by their molecular weight (500 to 20,000) which influences their activity: the greater the molecular weight, the greater the dispersion effect of the active molecule.
These polymers will be more stable at a given temperature than phosphonates (>300°C in general) and will present a very good resistance to chlorine.
the means of fighting corrosion
We can distinguish two principles for preventing corrosion:
- cathodic protection;
- anodic protection.
They block the corrosion process by opposing the migration of metallic ions towards the surrounding environment.
anodic inhibitors
the mechanisms and the application of anodic inhibitors:
Anodic inhibitors are chemical compounds which, via a reaction with metallic ions or with other ions (e.g. Ca2+), are capable of forming a protective deposit on anodic surfaces whilst opposing the migration of metallic ions.
The two possible mechanisms on the anodic surface are:
- oxidation of the surface of the metal to form a passivating film of metallic oxides;
- blockage of the electrochemical half-cell by precipitation of insoluble complex salts (eg : bicarbonate or iron phosphate).
This mode of action imposes the formation of a perfect film acting on all of the anodes. In the event of an “imperfect” film, there will be a loss of metal which will concentrate on the remaining anodes, resulting in pitting corrosion. It is therefore essential to permanently maintain a sufficient quantity of anodic inhibitor in the system in such a way as to prevent pitting. Anodic inhibitors must be used at high dose (in the order of g/L), often reserved for closed circuits.
anodic inhibitors
- nitrites ;
- silicates ;
- molybdates ;
- orthophosphates ;
- bicarbonates.
cathodic inhibitors
the mechanisms and the application of cathodic inhibitors
The cathode is a specific point in the system which means filming the entire surface of the installation is not necessary. Cathodic reactions are managed via the formation of a barrier made by the precipitation of insoluble salts, which physically isolates these zones from the water and the cathodic reaction to be blocked.
These inhibitors are safer than anodic inhibitors; localised corrosion does not occur in the event of under-dosing.
The doses to be implemented are low. Calcium carbonate is considered as a very good corrosion inhibitor. Calcium carbonate is formed and it precipitates and deposits a protective film. This reaction is very difficult to control. It is also dangerous as the scale reduces the thermal exchange. It is for this reason that other inhibitors are used.
cathodic inhibitors
Their use can be limited, owing to the concentration limits authorised in blowdowns. The main cathodic inhibitors are:
- zinc : very widely used, can be associated with phosphonates, must be stabilised to not precipitate during its injection;
- polyphosphates: can hydrolyse into orthophosphates ;
- orthophosphates: risk forming a too large deposit in the event of over-dosage or in the presence of a high THCa ;
- phosphonates : Depending on the phosphonates used, cathodic inhibition is more or less effective.
film-forming organic inhibitors
These organic compounds action is related to the formation of a fine continuous barrier which isolates the metal from the water.
This type of inhibitor has the particularity of being effective at acid pH (up to 4 ).
- fatty polyamines: they are sparingly soluble in water and exist in the form of emulsions. They can reduce the oxygen diffusion rate which proportionally reduces corrosion rate;
- aromatic amines : Benzotriazole, Tolyltriazole, Mercaptobenzotriazole : specific for the protection of copper but also of ferrous metals. Depending on the active molecules, there may be a sensitivity to oxidizing biocides.
preventing bio-fouling
biocides
definition and principles
Biocides are the active substances and preparations containing one or several active substances in the form they are delivered to the user, which are intended to destroy, repulse or render inoffensive harmful organisms, to prevent their action or combat them in any other manner via a chemical or biological action.
The use of biocides does not imply killing all the micro-organisms present and / or targeted. The action sought must be defined taking into account the environment in which it will occur. Disinfection is an operation with a temporary result permitting micro-organisms present on contaminated inert surfaces to be eliminated or killed and / or undesirable viruses to be inactivated, depending on the objectives fixed. The result of this operation is limited to the micro-organisms present at the time of the operation.
Before considering applying a biocide formulation, it is necessary to degrade growth conditions as far as possible.
Without further information, the injection of a biocide implies significant monitoring with the objective of assessing the effect truly obtained and the recontamination rate.
Warning: a biocide has no cleaning action.
oxidizing biocides
An oxidizing biocide is a formulation which will have an oxidizing action on other chemical compounds. These reactions implement oxidizing elements such as Chlorine, Bromine, Oxygen. The action at the level of the microbial cell results in the perturbation or inhibition of vital metabolisms.
The main characteristics of oxidizing biocides are:
- a fast action rate of between 1 and 15 minutes depending on water quality;
- a continuous injection;
- an action spectrum which concerns all oxidizable matter and not only micro-organisms;
- doses according to the micro-organism, contact time, water quality;
- an oxidizing power which can influence corrosion;
- they can be inactivated.
Due to their more or less significant reactivity, these formulations can also be used for other actions such as iron removal, degrading or oxidizing troublesome molecules, colour removal etc.
principle of use:
The principle of use consists in permanently maintaining the residual of oxidant necessary to obtain the desired quality of water from a microbiological viewpoint. Their great reactivity and the diversity of molecules with which a chemical reaction is probable, mean that it is not possible to know the order of reactions, nor at what moment the biocide action will occur among all of the possible reactions. It is for this reason that it is necessary to determine the “oxidant demand” of a given water.
the substances most often used
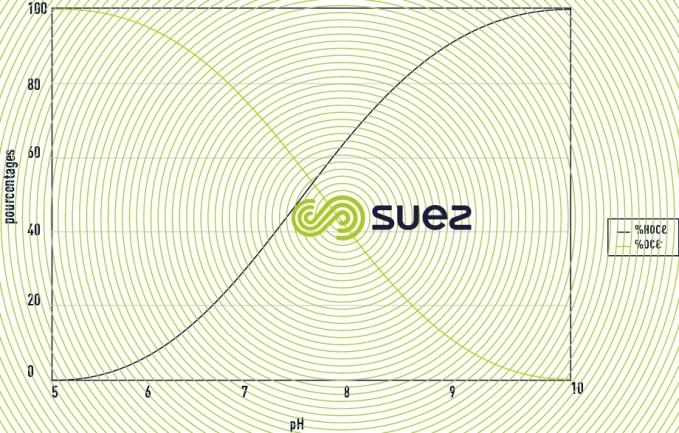
- the precursors of hypochlorous acid: these are the formulations, which in an aqueous environment, will free hypochlorous acid: HOCl. It consists in gaseous chlorine (Cl2), bleach extract (NaOCl + NaCl), calcium hypochlorite (CaOCl), halohydantoins (BCDMH), “cyanurates” (ATCC - DCCNa). The additives which generate hypobromous acid are included in this family. In water, hypochlorous acid will be balanced with the hypochlorite ion according to the reaction below.


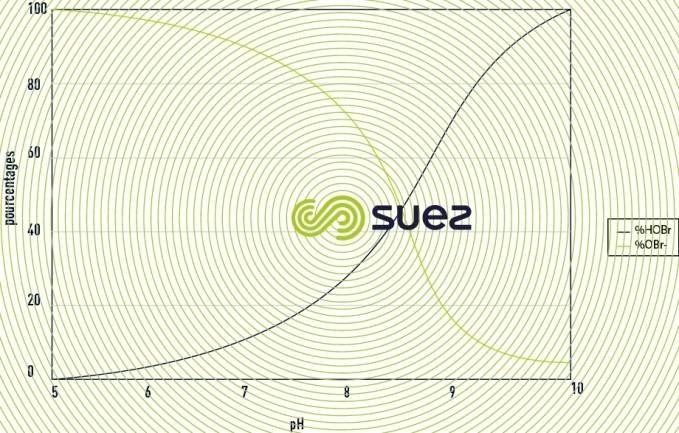
The proportion of each molecule will depend on the value of the pH as represented in the figure 32. For brominated products the principle is identical but shifted on the scale of pH as we can see in the figure 33. Hypochlorous acid and hypobromous acid can also intervene in addition or substitution reactions. These reactions represent a potential consumption of active substances.
- active molecules: Only hypochlorous acid and hypobromous acid have a known biocide activity. The higher the pH, the greater the quantity of precursors to be implemented.
- chloramines and bromamines: Halogenamines (chloramines and bromamines), also known as “linked or stabilised or even combined Chlorine or Bromine” as opposed to free Chlorine or Bromine. These formulations have a longer shelf-life. However, once in the water, not all of the hypochlorous acid in the product will be released immediately. The speed of release will depend on the product and water quality. This makes product activity more complex to manage given that an increase in the dose will not result in a proportional increase in the effect.
- benefits of bromination: The dissociation of hypobromous acid in water with respect to pH is similar to that of hypochorous acid, except that the phenomenon is shifted on the scale of pH and gives an advantage to Bromine for increasing pH values. The oxidising power of bromine is close to that of chlorine.







- chlorine dioxide is highly soluble in water. Its concentration in the solution depends on temperature and pressure. Furthermore, it is volatile and can therefore be easily eliminated by aeration. In water, chlorine dioxide has almost no chemical reaction. Its activity will not depend on pH value. Chlorine dioxide is a gas which is difficult to transport; it is often prepared in-situ. The most frequently used method is a proportionate mix of diluted solutions of hydrochloric acid and sodium chlorite. The oxidizing power of chlorine dioxide is comparable to that of chlorine. Chlorine dioxide will only react by oxido-reduction and in this capacity, will be less reactive as regards organic matter than bleach extract. Chlorine dioxide does not generate chloramines or any other organochlorinated compounds, including trihalomethanes (THM).


- “oxygen” precursors: when stored, formulations are often not very stable. They are highly reactive, but not all of the reactions have a “biocide effect” which constitutes a potential loss as regards this effect. Among the most frequently used active substances, we can mention:
- hydrogen peroxide;
- peraceticoacid;
- ozone which requires a generator as this gas cannot be stored and must be produced in-situ.
non-oxidizing biocides known as « synthetic biocides »
Synthetic biocides consist of formulations which are not considered to be oxidizing biocides.
These products are generally used when conditions do not allow the use of oxidizing biocides.
The main characteristics of non-oxidizing biocides are:
- action time of between 30 minutes and 24 hours;
- continuous or discontinuous injection;
- more or less specific spectrum (algicide, bactericide, fungicide);
- doses according to the micro-organism, contact time, water quality;
- can be inactivated.
principles of use
Synthetic biocides can be used in continuous dosing or “surge dosing”. The injection of a surge dose over a short duration allows a significant concentration to be periodically reached and if necessary, a more intense effect to be obtained in zones which are difficult to reach such as bio-films.
The frequency of surge doses is a balance between the applied dose, in other words the desired effect, and the rate of circuit recontamination. Recontamination will depend on environmental data which will imply that the circuit is more or less a favourable breeding ground for microbes.
the substances which are most often used.
this family of products typically includes:
- organosulphur compounds (Isothiazoline, thiocarbamate);
- organobrominated compounds (BNPD, DBNPA);
- quaternary ammoniums, Aldehydes (Glutaraldehyde).
This list is not exhaustive and concerns the active substances most commonly encountered in the water treatment domain.
particular case of on-site generation
It is important to evoke this type of technology which provides a response to problems linked to the limited conservation of certain oxidizing biocides, or the storage of hazardous products.
generation of chlorine dioxide
This technique is relatively old and well- managed. For this, it is necessary to use a generator which is a secured apparatus for the mixing of 2 precursors, the most often hydrochloric acid and sodium chlorite, but it can also be sulphuric acid and sodium chlorite, or gaseous chlorine and sodium chlorite. It is recommended to use diluted precursors. Precursors must not be placed in contact outside of the generator, which imposes compliance with certain safety rules.
generation of hypobromous acid
To produce hypobromous acid it is necessary to mix bleach extract and sodium bromide. To determine whether it is necessary to use this technology it is necessary to calculate the technical-economic benefits, in other words, to compare the concentration of active substance for a given pH with the cost of the application. Two cases present an interest depending on pH value: 100% HOCl or 100% HOBr. The other mix ratios will be less effective for a same cost, or more expensive for a same effectiveness. This technology presents a technical-economic benefit when the pH of the water to be treated exceeds 8.5. For lower pH values, the benefits need to be studied on a case-by-case basis. By generating 100% of hypobromous acid we will have, for the same pH, a greater proportion of ACTIVE free oxidant than if we were to use hypochlorous acid (see figures 32 and 33).
The product synthesis must occur on site and the mixture must be immediately injected into the water to be treated as this mix is not stable over time and in a few hours, loses its effectiveness.
generation of monochloramine
This technology produces a hypochlorous acid (HOCl) precursor to be used in the event of a very high demand in oxidant for a water to be treated. Consequently, the product activity is more focused on the biocide action and the losses of active substance due to the great reactivity of the HOCl are limited. This technology could be envisaged for applications in the iron and steel industry, or for water which is extremely contaminated by organic matter.
The product synthesis must occur on site and the mix must be immediately injected into the water to be treated as it is not stable over time and in a few hours, loses its effectiveness. Mixing conditions: the precursors used must be known and mastered to avoid any risk of de-gassing or exothermic reactions.
generation of chlorine by electrolysis
This technology implements a non-hazardous precursor (sodium chloride) which is very stable over time. This considerably reduces the risks linked to the use of chemical products if we compare with biocides in general, or with bleach extract or gaseous chlorine. This stability and security result in a significant fall in costs. The chlorine produced passes into water in the form of hypochlorous acid. Compared with bleach extract, there are no associated by-products such as salts, chlorates etc.





physical techniques
ultra-violet rays
UVs are rays which are specifically produced by lamps which must be sized in terms of power and therefore, according to the effect sought in view of water quality. The parameters to be considered are suspended solids, transmittance, microbial contamination, concentration in oxidizable matter. Maintenance work (cleaning and changing lamps) is to be anticipated. Several types of UV exist; only UVcs have a biocide effect. They are sensitive to water temperature: optimum 20°C. The action of UVs occurs at the level of the DNA of micro-organisms. Automation and adaption to the change in water quality is very difficult and complex. UVs do not result in any (or few) modifications to water quality.
Whilst the use of ultra-violet rays can reduce the use of biocides, as the technology exists today it cannot replace them.
Even though the effectiveness of UVs no longer needs to be proven, their action remains localised to the position of a lamp on a circuit and biocide activity cannot be “exported” or transported towards distant contaminated zones.
ultrasound
Ultrasounds are a physical vibration produced by an electric current in a chamber. This vibration will be characterised by its amplitude and frequency. Depending on these characteristics, we will produce air bubbles or void which will be de-structuring. It is these vacuum bubbles which will be more effective and will enhance biocide effect. The action consists in de-structuring and disorganising the cellular cohesion of bacteria. As with UVs, the installation (reaction chamber) must be sized according to water quality. The parameters to be considered are suspended solids, transmittance, microbial contamination, concentration in oxidizable matter and in organic matter.
Whilst the use of ultrasound can reduce the use of biocides, as the technology exists today it cannot replace them.
Even though the effectiveness of ultrasounds no longer needs to be proven, their action remains localised to the chamber on a circuit and biocide activity cannot be “exported” or transported towards distant contaminated zones.
The use of ultrasounds can result in a rise in the temperature of treated water of approximately 1°C.
related techniques: surface-active products
The terms typically used for these formulations are bio-dispersants and bio-detergents. It consists of a family of products with various properties. Bio-dispersants or bio-detergents do not generally possess a biocide effect. Formulations are surfactants and modify the physical characteristics of the treated water by changing the superficial tension exerted on surfaces.
A surfactant molecule includes two parts:
- a polar group which possesses an affinity for water (hydrophilic);
- a non-polar group (hydrophobic) or slightly polar group which possesses an affinity for fatty bodies.
The active substances are classified according to the nature of the polar component:
- anionic;
- non ionic;
- cationic;
- amphoteric.
The structure of surfactants gives them the following properties:
- electrochemical: anionic or cationic molecules reacting with opposite charge sites. This property contributes towards increasing the wettability of deposits and weakening them mechanically;
- solubilisation or hydrotropic: action aiming to make a soiled element or part of it soluble. Bio-films can contain little soluble or non-soluble elements such as lipids or proteins. The active substances used will have a hydrophilic pole and a hydrophobic pole;
- wetting: contributes towards increasing the penetrability of water within a complex organic structure. The action is concretised by a modification of the superficial tension (an increase in the spreading surface of a water droplet);
- dispersant: to avoid agglomeration and maintain suspension;
- detergent: process involving the “de-coupling” of an element from its support.
These properties are exploited to eliminate the bio-film or slow down its formation in cooling towers. They can be complementary to the biocide action and allow biocides to have an action on difficult to access zones.
To be fully effective, a surfactant treatment must be associated with a mechanical effect generally produced by the water flow. The start-up of a surfactant treatment can contribute towards weakening bio-film structures and consequently put a more or less significant flora, which until that time had been fixed, back into circulation. These periods must be the focus of increased surveillance and a reinforcement of the associated biocide treatment.
When a surfactant effect is required via the use of a bio-dispersant or bio-detergent, the product must comply with the requirements of ruling n° 648-2004, modified by the EU ruling 907-2006, relating to detergents.
The choice of treatment strategy includes regulatory constraints in particular, the texts relating to the environment, water, and biocide discharges.
Bookmark tool
Click on the bookmark tool, highlight the last read paragraph to continue your reading later










