substrate properties
Reading time:The term substrate refers to all products contained in water and likely to be used by bacteria for growth. These elements can be classified as follows:
- major elements: C, H, O and N;
- minor elements: P, K, S and Mg;
- vitamins and hormones;
- trace elements (Co, Fe, Ni…).
In the particularly complex medium that includes most wastewater, trace elements, vitamins and hormones are usually present in concentrations that are sufficient for satisfactory water treatment. The same applies to K, S and Mg. On the other hand, the water to be treated might not contain sufficient phosphorus or even nitrogen. This is typical of some IWW (even when combined with UWW ); phosphorus and nitrogen then have to be added.
On the other hand, the fight against eutrophication may require these elements to be eliminated (see suspended growth (activated sludge) processpage 3).
carbonaceous pollution
In general, carbonaceous pollution is the main pollution that needs to be eliminated. It is also the main constituent of biomass (very simplified formula: C5H7O2N or 53 % of C).Given the various forms of carbonaceous pollution, we generally have recourse to global characterization methods, i.e. (see terms used by water analysts):
- biochemical oxygen demand ( BOD);
- chemical oxygen demand ( COD);
- total organic carbon (TOC).
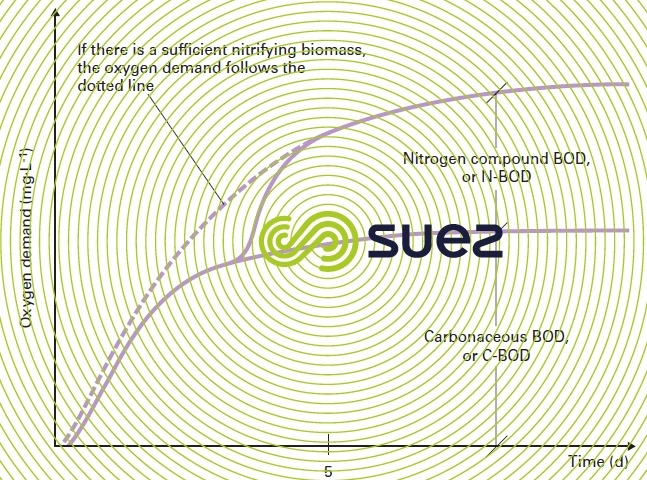
Figure 4 shows the change in BOD measured from the oxygen consumed by a bacterial culture. In order to be complete, this change requires approximately three weeks at 20°C. We then obtain ultimate BOD or BOD21.
If, in a water, all organic matter was biodegradable, we should have:

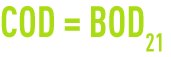
This applies to glucose where we have

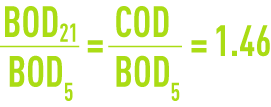
(please refer to the formulary, section characteristics solution constants for additional examples).


When there is non-biodegradable organic matter (domestic water and many types of industrial discharge), we have:


As typical examples of non-biodegradable organic compounds, we can quote cellulose, lignin, tannins…
During a biological treatment, the effluent’s


ratio increases noticeably and will do so increasingly when purification is more intensive.
Figure 4 also shows the error that is committed if we confuse the oxygen that is actually consumed to eliminate carbonaceous pollution with the oxygen consumed by the nitrification of the effluent’s organic or ammonia nitrogen.
nitrogen pollution
In "fresh" wastewater, nitrogen is mainly found in proteins and urea but, during the wastewater’s passage through the sewers, a significant proportion of the organic nitrogen is hydrolysed to form ammonia. As it enters the plant, nitrogen will typically be found as ammonia (60 to 75%) and organic nitrogen (25 to 40%) in a soluble and particulate form.
The sources of nitrogen that are likely to be used by the various micro-organisms (see the nitrogen cycle) include practically all sources of organic and mineral nitrogen. Nitrogen is metabolised to provide mainly proteins, nucleic acids and cell wall polymers. It is understood that nitrogen constitutes approximately 12.4% of the dry weight of a pure biomass. In wastewater treatment, this figure is usually less than 10%, typically between 5 and 10%.
phosphorus pollution
Phosphorus is present in wastewater either as orthophosphates or as polyphosphates or organic phosphorus.
This phosphorus is mainly found in nucleic acids, phospholipids and polymers in bacteria walls. In some individual cases, this phosphorus can be stored in the cell under different forms (biological phosphorus removal, see suspended growth (activated sludge) process)
Excluding biological phosphorus removal, phosphorus represents 1.5 to 2.5% of the biomass’s dry weight. However, it should be noted that this percentage will rise as the growth rate rises and that it varies in inverse proportion to temperature.
Bookmark tool
Click on the bookmark tool, highlight the last read paragraph to continue your reading later













