preparation
Reading time:Chlorine dioxide is always prepared as a solution on site via oxidation of sodium chlorite using chlorine or hydrochloric acid (see chlorine dioxide).
In both cases, the pH where the reagents are mixed needs to be very acidic to avoid secondary reactions that result in the rapid formation of chlorate ions.

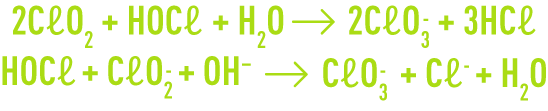
The secondary formation of chlorate ions also depends on the chlorite concentration present in the reaction medium; that is why the chlorine and chlorite concentration ratio is another determining parameter in the production process.
The process using acidification requires 1.25 times more sodium chlorite and is, therefore, more expensive. The choice to be made between the two production options will, therefore, be dictated by the availability of chlorine storage facilities. Both processes call for special preparation and safety provisions (see chlorine dioxide).
Bookmark tool
Click on the bookmark tool, highlight the last read paragraph to continue your reading later












