carbon dioxide CO2
Reading time:Carbon dioxide is used for remineralisation in drinking water applications, combined with lime, and also for neutralisation (see reagent and treatments than can be used). In neutralisation, carbon dioxide is used to achieve precise pH adjustments through fine-tuned amounts and through a neutralisation curve that is more gradual than that achieved with strong acids.
In remineralisation, approximately 9 g of CO2 and 7.5 g of Ca(OH)2 will be needed to provide 1 m³ of water with 1 degree of TAC.
principle of a CO2 injection unit
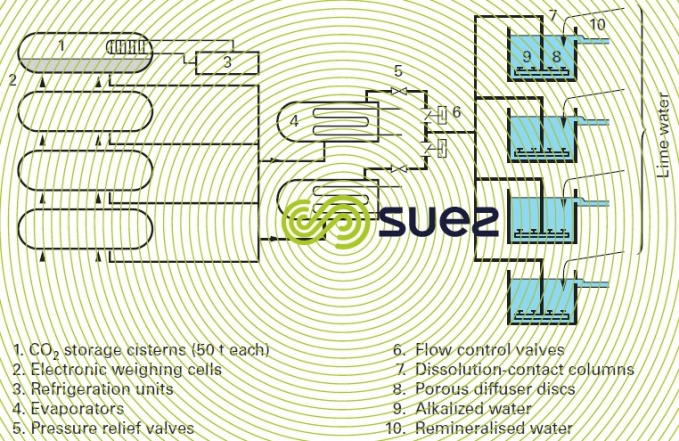
Although carbon dioxide can be produced with burners that may or may not be submerged in the water to be treated, the most widespread method used is CO2 stored as a liquid in pressurised, lagged and refrigerated tanks (20 bar pressure, -20°C temperature). When liquid CO2 is drawn off, it is vaporised in evaporators (requiring an external heat source) and then expanded to utilisation conditions (3 bar at 12°C). It is finally injected as a gas into the water to be treated through porous diffusion discs submerged below several metres of water in the contact column, or injected by means of an injector (see chlorine injection operating principle).
When low flow rates are required, CO2 can be stored in cylinders.
layout example
Figure 17 applies to the drinking water remineralisation unit in the town of Lisbon-Asseiceira (Portugal).
The installation’s total capacity can be adjusted from 200 to 850 kg · h–1 of CO2. The system’s output is governed by the throughput of water to be treated, by target treatment level and, possibly, by remineralised water pH. This adjustment is carried out using control valves mounted on the gas circuit; in turn, these control valves are controlled by controllers that receive signals from the process’s programmable logic controller.


Bookmark tool
Click on the bookmark tool, highlight the last read paragraph to continue your reading later












