corrosion in metal: local cell model
Reading time:Our understanding of corrosion caused to metal by water has been systemised using the localised corrosion cell model. This model provides a description of the electro-chemical process that causes metals to dissolve. This model is based on the Wagner & Traud "mixed potential theory" with its two principles :
- every electrochemical reaction can be broken down into at least two partial oxidation and reduction reactions ;
- there can be no net accumulation of electrical charges during an electrochemical reaction.
In this model, metal destruction through oxidation and dissolution is shown as the following reaction :


This oxidation reaction has been defined as the "anodic" process. In order to meet the electro-neutrality requirement, the oxidation and reduction reactions must take place at the same time, thus consuming the same number of electrons :


The reduction reaction is called the "cathodic" process. The combined oxidation reduction process, at equilibrium, is written as :


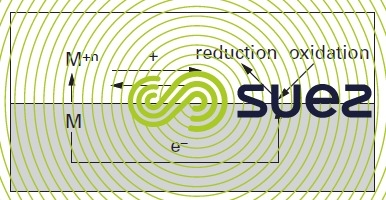
Figure 1 illustrates the overall process.
The global potential of the combined oxidation reduction process will determine whether or not the reaction can take place and, consequently, the likelihood of corrosion.


Bookmark tool
Click on the bookmark tool, highlight the last read paragraph to continue your reading later












