the physical states of impurities in water
Reading time:The water found in the natural world is never pure, and this is even more so in the case of treated water. Its impurities come mainly in two states:
suspended solids (figure 4)
Mineral or organic materials that remain suspended due to the water’s turbulence or because their density is too close to that of water. They do not offer any major interference in relation to the water surrounding them (except for buoyant force, which is a major factor in clarification and flotation phenomena. (See fundamental physical-chemical engineering processes applicable to water treatment).

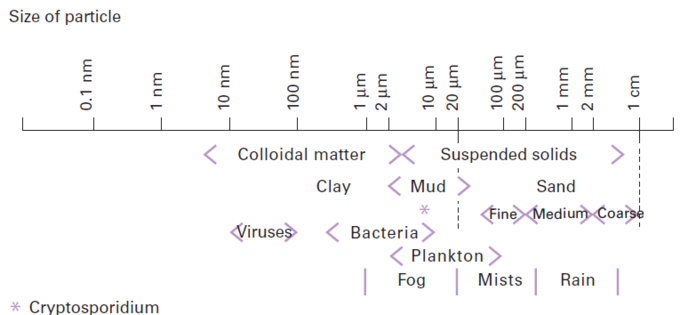
Of these, colloidal suspensions feature very finely-divided solids (0.01 to 5 µm) characterised by a major specific surface area (cm²/g) and an electrostatic charge that is generally negative and which builds up at the solids/liquids interface (see Zeta potential, general comments). These suspensions, which are often solids, can also consist of a liquid that is non-miscible in water (for example, oil globules suspended in water, etc.). In this case, the term used is mechanical emulsion if what is involved is an emulsion that is not stabilised by a chemical product, or chemical emulsion if the emulsifiers at the water/non-miscible liquid interface prevent any coalescing of the droplets and therefore make the emulsion highly stable (for example “soluble oils”).
dissolved substances or true solutions
These involve mineral compounds (generally ones that are ionised to a greater or lesser extent) or organic compounds, whether or not they have macromolecules, as well as gases that are often highly water-soluble (for example CO2, SO2, NH3, etc.).
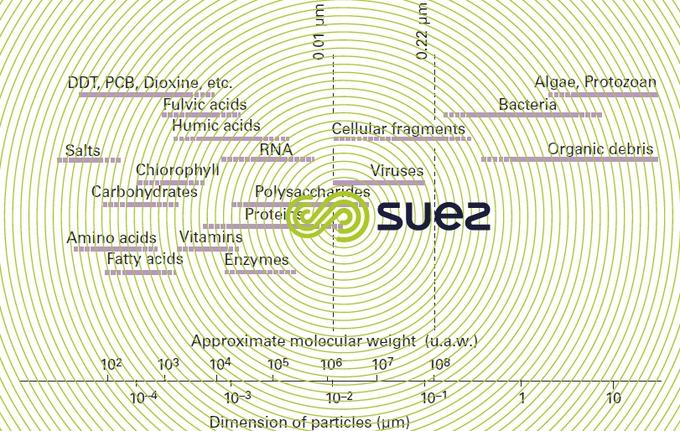
Figure 5 gives an idea of the sizes and molecular weights of the dissolved and suspended compounds in natural water or wastewater.


Bookmark tool
Click on the bookmark tool, highlight the last read paragraph to continue your reading later












