ionisation
Reading time:A mineral compound that is dissolved in water dissociates to a greater or lesser extent upon the appearance of negatively-charged ions (anions) and positively-charged ions (cations).The dissolved substance is called an electrolyte; it carries the electric current.


When several electrolytes are in the same solution, each one dissociates to a certain extent, and the ions released can inter-combine to form new compounds. For example, if you dissolve two compounds AB and CD, in solution you will find molecules AB, CD, AD and CB in equilibrium with the A+, B-, C+ and D- ions. This equilibrium can be changed (Le Chatelier’s law) if insoluble compounds, complexes or gases are formed. If, for example, compound AD is insoluble, the equilibrium will shift almost completely to the right, according to the reaction:


Even in a relatively concentrated solution, acids and strong bases, as well as their salts, are completely dissociated. They are called strong electrolytes (for instance, nitric acid, sulphuric and hydrochloric acid, caustic soda and potash bases, and sodium chloride salt).
Other substances, such as acetic acid CH3COOH, hydrogen sulphide H2S, and phosphoric acid, are only partly dissociated in solution. These are weak electrolytes. The polyelectrolytes used for clarification (among other things), and which feature several ionisation sites, fall within this category. A distinction is drawn between total acidity, which includes all the potential H+ ions, and free acidity, which includes free H+ ions.
Water itself experiences partial ion dissociation, in accordance with the following reversible reaction:

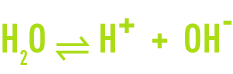
The H+ proton does not exist in a free state in an aqueous solution. Each hydrogen ion combines with a water molecule to form the hydroxonium H3O+ ion, a hydrated proton.

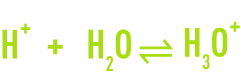
Thus, in water you find H2O molecules, OH- hydroxide ions, and H3O+ hydroxonium.
the law of mass action
Assuming a chemical reaction in a state of equilibrium:

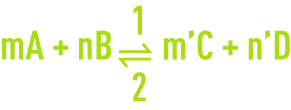
The reaction speeds in directions 1 and 2 are provided by the relationships:

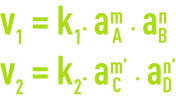
aA, aB, aC and aD represent the activities of compounds in solution.
At equilibrium v1 = v2, hence:

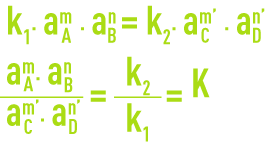
K is called the thermodynamic dissociation constant. For greater convenience, the following notation is used:

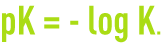
A table (see characteristics solution constants) provides the pK values at 25°C for standard acids and bases.
application of the law of mass action to water: the concept of pH
Assuming that the activity coefficients equal 1 (a valid hypothesis for highly diluted solutions), the formula used is:

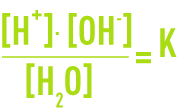
As the dissociation is still weak, the concentration of the water molecules is practically constant and the following can be written:


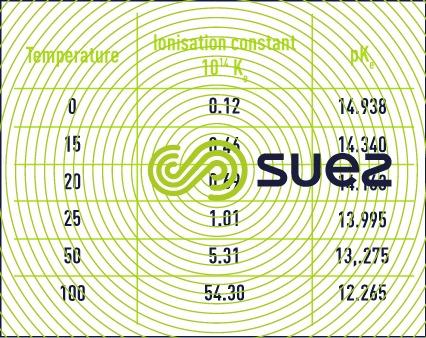
The dissociation constant (or ionic product) for water is approximately 10-14 (mol · L-1)2 at 25°C. It varies depending on the temperature (see table 7).
In pure water,

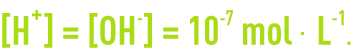
An aqueous solution’s pH is defined based on the mole concentration in terms of H+ ions in accordance with:

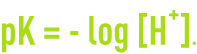
The pH is measured using the electrometric method (glass-electrode pH metre).


A solution in which the pH < 7 is called an acid medium, while a solution where the pH > 7 is called a base medium.
the strength of acids and bases in aqueous solution
An acid is a substance capable of losing protons, or in other words H+ ions. A base is a substance capable of accepting these protons. In aqueous solution, there is a conjugated acid-base couple which is defined by the following equilibrium:


By applying the law of mass action to a highly-diluted solution where [H+] = [H3O+] you obtain:

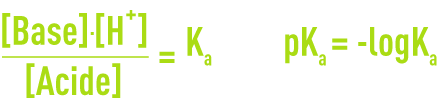
Thus defined, Ka is called the ionisationconstant for the acid-base couple.
For example, acetic acid in water gives rise to a pKa equilibrium of 4.8:


The more H+ ions it provides in water, the stronger an acid is, in other words the higher Ka is and the weaker pKa is. The higher the pKa, the stronger the base.
Thus, the NH4+ ammonium ion is a weak acid where pKa = 9.2 at 25°C. The corresponding NH3 base (NH4OH in an aqueous medium) is a weak base.
salts hydrolysis
Salts can be placed in four categories, depending on the strength of the acids and the bases they come from:
- strong acid and strong base (for example: sodium chloride);
- weak acid and strong base (for example: sodium acetate);
- strong acid and weak base (for example: ammonium chloride);
- weak acid and weak base (for example: ammonium formate).
Aqueous salt solutions in the last three categories can be neutral, acid or base, depending on the interactions with the ions from the water. For example, for a salt AB from a weak acid AH and a strong base BOH, the dissociated A- anionscombine with the protons resulting from the ionisation of water to provide the non-dissociated acid AH. Consequently, the hydroxide ions concentration increases in the solution, which becomes a base. The overall equation is:


For example, hydrolysis of sodium acetate at a concentration of 0.01 mol · L-1 results in a pH solution of 8.4:


Category one salt solutions are still neutral as the corresponding acids and bases are strong electrolytes.
the pH of aqueous solutions
The pKa concepts allow the pH for acid solutions, base solutions, or salts with a molar concentration of C to be calculated. At 25°C:
- the pH for an acid solution is:

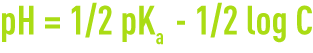
- the pH for a base solution is:

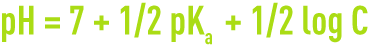
- the pH for a salt solution is:
- for a salt with weak acid and a strong base,

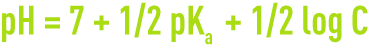
- for a salt with strong acid and a weak base,

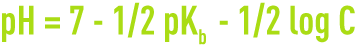
- for a sale with weak acid and base,

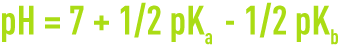
With Ka and Kb being the ionisation constants for the corresponding acid and base.
the solubility of sparingly soluble salts. Solubility product
Experience shows that in a saturated solution of a salt AB that is sparingly soluble (solubility lower than 0.01 mol.L-1), the product of the ionic concentrations [A+]·[B-] is constant for a given temperature and ionic force for the solution. This product, the notation for which is KS, is called the solubility product.
The less soluble the substance is, the weaker the value of KS is. For soluble calcium carbonate at a rate of 12 mg · L-1, the solubility product KS equals 10-8.32 (mol · L-1)2. By analogy, with the pH, you write pKS= -log KS.
The presence of other salts increases the solution’s ionic force, and consequently this changes the solubility. The solubility increase, called the salt effect, is marked when the electrolyte does not have a shared ion with the salt in question. Otherwise, the solubility decreases. For example, in a 0.01 mol.L-1 solution of sodium chloride, silver chloride’s solubility is divided by 1,000.
buffer solutions and buffer power
A buffer solution is a solution with pH that shows little variation when small quantities of acid or base are added. These solutions are of practical use when you need a reaction to occur at a constant pH level. These are obtained by mixing a weak acid and its sodium or potassium salt or a weak base and its strong acid salt. For example: acetic acid – sodium acetate (pH = 3.7 to 5.6), sodium hydrogen orthophosphate – sodium di-hydrogen orthophosphate (pH = 6.0 to 9.0), etc.
Bookmark tool
Click on the bookmark tool, highlight the last read paragraph to continue your reading later












