oxidation-reduction-speticity potential
Reading time:Household wastewater that is sufficiently "fresh" will have an oxidation reduction potential of approximately 100 mV which, for a pH in the region of 7, produces an rH of 17 to 21 (see oxidation-reduction). A potential of less than + 40 mV (i.e. rH = 15 for a pH = 7) or a negative potential will be characteristic of a reducing medium (septic water, putrid fermentation, presence of reducing agents). A potential of more than 300 mV (i.e. rH = 24 for a pH = 7) is indicative of an abnormal oxidising medium (large amounts of water entering the system: rain – spurious water).
In the presence of SO42‑, the effluent’s septicity will result in the formation of sulphides (S2-) and cause H2S, as well as corrosion, to be emitted to the network.
Figure 22 classifies water according to pH and rH.

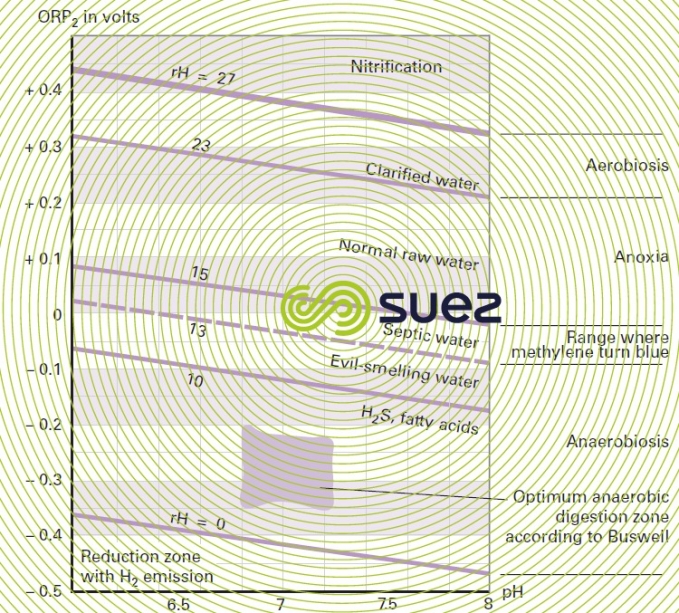

It should be noted that 4 factors play an important part in sulphides production in a network:
- temperature: below 15°C, it is rare to find values above 2-3 ppm S2-;
- SO42- concentration in the water because most of the S2- is created through the action of sulphur reducing bacteria;
- pollution concentration: in fact, the higher the BOD, the faster anaerobiosis takes place providing sulphur reducing bacteria with more "food";
- water dwell time in the networks and, therefore, the length of these networks and flow velocity through said networks.
Thus, on the longer networks (dwell time > 12 h) and at temperatures of 20°C, we go from between 5 and 10 ppm S2- to between 20 and 30 ppm at temperatures of between 25 and 28 °C, and even from 50 to 70 ppm in the case of prolonged transfers taking approximately 24 hours in hot countries when water contains more than 300 ppm SO42-.
Bookmark tool
Click on the bookmark tool, highlight the last read paragraph to continue your reading later












