phosphorus cycle
Reading time:The presence of P in water is due to:
- natural sources (erosion, leaching)
- to diffused (fertilisers) or momentary (effluent, especially in conjunction with detergent discharges with which phosphates are often connected) pollution.
In terms of P speciation, we have the following forms:
- dissolved minerals (orthophosphates, polyphosphates);
- dissolved organics ( DOP );
- mineral particulates (apatites, ferric phosphates, phosphates that are adsorbed over suspended solids);
- organic particulates (cell matter such as DNA, RNA, ATP; faecal pellets etc.).
The P cycle in natural water is shown in figure 5; the particular features of the biochemistry applicable to this element in effluent will be examined along with biological phosphate removal treatments (suspended growth (activated sludge) process).

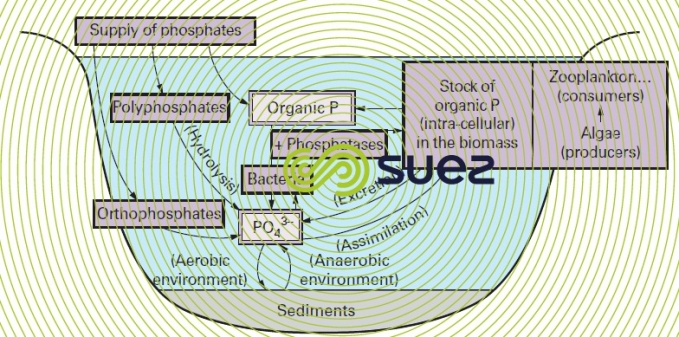

In order to develop, algae and bacteria mainly use PO43- ions (their ability to be dissolved in water can be caused by microbial action, especially due to the production of mineral or organic acids); nevertheless, they are also capable of assimilating the DOP thanks to specific enzymes called phosphatases that will be acid or alkaline depending on the pH at which they achieve their maximum efficiency.
During their lifetime or after their death, aquatic organisms discharge organic (particulate or dissolved) or mineral P; all these forms can be recycled immediately or after bacterial mineralisation, using the various mechanisms described above.
Additionally, there are two P cross flows between water and sediments:
- precipitation under aerobic conditions (as apatites, ferric salts, etc.);
- solubilisation under anaerobic conditions (phosphates reduced to phosphites and phosphines, solubilisation of ferrous iron).
Stocks of P held in the biomass and in sediments maintain eutrophication conditions in water sheets (see pollution and eutrophication).
Bookmark tool
Click on the bookmark tool, highlight the last read paragraph to continue your reading later












