composition of the material
Reading time:We call pure substance a substance whose physical constants remain identical regardless of the sample collected size.
The molecule is the smallest part of a pure substance that retains all the chemical and physical properties of the pure substance.
A molecule can be disintegrated using chemical or physical ways. High levels of energy are used. The molecule is formed of atoms that are joined by chemical bonds :
- identical atoms for a simple pure substance, or
- different atoms for a composed pure substance.
The atom is the smallest conceivable part of a chemical element. It consists of a positively charged nucleus around which negatively charged electrons gravitate. The nucleus is formed of two types of nucleons: protons and neutrons (figure 5) :

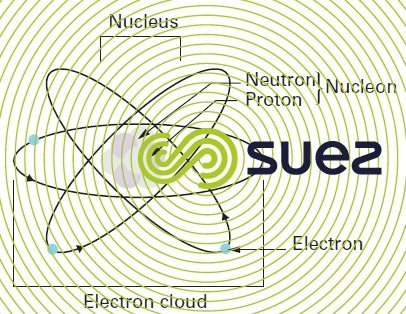

- the electron has an electrical charge

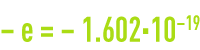
coulomb. Its mass is 9.109·10–31 kg. The electron has an angular momentum (spin) caused by its rotation round its axis; this creates a magnetic dipole moment that is responsible for the properties of most para and ferromagnetic substances ;
- the proton is a particle that has a positive charge equal to

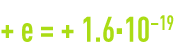
coulomb and its mass is 1 835 times that of the electron (1.673·10–27 kg) ;
- the neutron is an electrically neutral particle that has a mass that is 1.001 4 times that of the proton.
In space, the atom occupies a volume that can be compared to a sphere having a diameter of a few angströms. Its mass is approximately 10–26 kg. The nucleus has a diameter of approximately one fermi (10-15 m), that is to say very small compared to that of the atom. It concentrates virtually all the atom’s mass. The radius of the atom is 100,000 greater than that of the nucleus. An atom is identified by :
- its atomic number Z which refers to the number of protons in its nucleus;
- its atomic mass number A that is equal to the sum of the proton numbers Z and neutron numbers N.
The number of electrons equal to the number of protons Z determines the properties of the atom. Electrons move very rapidly within the nucleus’ field of attraction. They move on different orbits centred round the nucleus; these orbits refer to varying levels of energy that are defined by quantum numbers. Electrons can move from one level of energy to another according to rules, by absorption or emission of a certain energy that is a whole multiplier of a unit of energy called a quantum or a photon. The energy for the transition between two adjacent levels is equal to hu where h is the Planck constant (6.626 075 5·10–34 J·s), and where u is the frequency of the associated photon, inversely proportional to its wavelength l (l = c/u where c, speed of light constant, 299 792 458 m · s–1).
With the exception of noble gases that are monoatomic, matter in the gas, liquid or solid state is not formed of isolated atoms but of atoms that are grouped in molecules, in metal or ionic solids. This grouping is the result of bonds and interactions between atoms via the electrons in the outer layers, thereby creating stable structuress.
ions – isotopes
Ions derive from atoms through the loss or gain of electrons under the effect of added thermal or electrical energy. Electropositive elements such as iron produce positively charged ions known as cations. Electronegative elements such as chlorine and sulphur produce negatively charged ions known as anions. An ion can be formed from several electrically charged atoms (case of carbonate). In general, anions and cations do not exist in isolation but in groups as ionic compounds.
The isotopes for the same element refer to atoms carrying the same number of electrons but different numbers of neutrons. Therefore, isotopic atoms have the same chemical properties. They only differ through their kinematics.
Several isotopes are known for most of the elements. An isotope’s elements are distinguished by designating them by their symbol preceded by numbers A and Z :


atomic mass of elements – mole – Gramme-molecular weight
Because the electron mass is negligible, the mass of an atom is equal to the mass of the nucleons. As these are very small masses and, therefore, not that convenient to use, by convention, the unit of atomic mass (u) is defined as 1/12th of the mass of the carbon 12C atom (1.66054·10–27 kg) (14th General Conference on Weights and Measures 1971).
The Anglo-Saxons and biochemists use the dalton (Da) where 1 u = 1 Da. An element’s atomic mass is calculated as the sum of the masses of the different isotopes weighted for their relative abundance (e.g. the atomic mass of chlorine is 35.453 u).
Faced with the very large number of particles contained in a sample of matter, chemists created the mole which is the unit of measurement applicable to a quantity of matter (symbol "mol"). By definition, the mole is the quantity of matter in a system containing as many elementary entities as there are atoms in 12 g of carbon 12C.
The number of elementary entities in one mole of substance is 6.022·1023 (Avogadro number).
The mass of one mole of carbon 12C is 12 g precisely, that of one mole of chlorine atom is 35.5 g; the quantity of matter in 1 kg of water H2O is 55.533 mol.
The gramme-molecular weight of a substance is defined as the quotient of the mean mass of this substance by the quantity of matter it contains: it is expressed in grams per mole (g·mol–1). The molar mass of a molecule that has several atoms is usually calculated as follows :

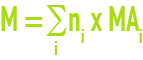
where ni is the number of atoms i with the atomic mass MAi.
element classification - formulae for the compounds
Chemical elements do not have unspecified properties; their properties depend on the atom’s electronic structure: two different atoms having identical peripheral layers will have similar chemical properties (alkali, halogen families …). The periodic classification of elements derived from the Mendeleev table (figure 6), takes these similarities into account. In this table, each element is represented by a symbol and an atomic number. The mean atomic mass of each element is shown in the bottom right hand corner of its box.

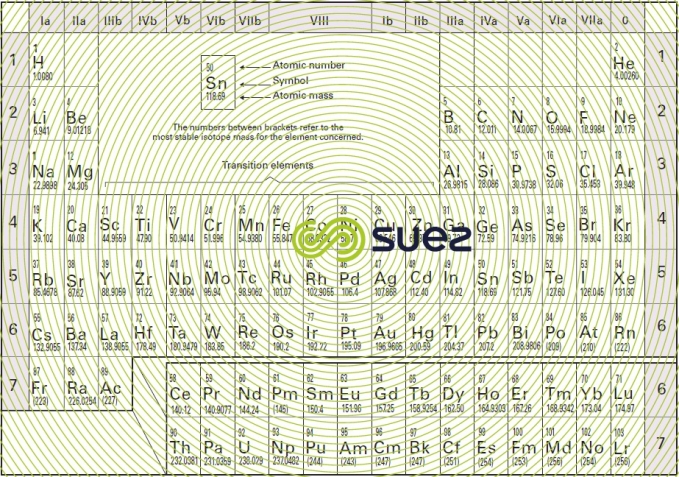

Chemical substances are represented by formulae obtained from the symbols of the elements which constitute them. Each symbol is allocated an index number showing the relevant number of atoms. The H2O water molecule contains 2 atoms of hydrogen and 1 of oxygen.
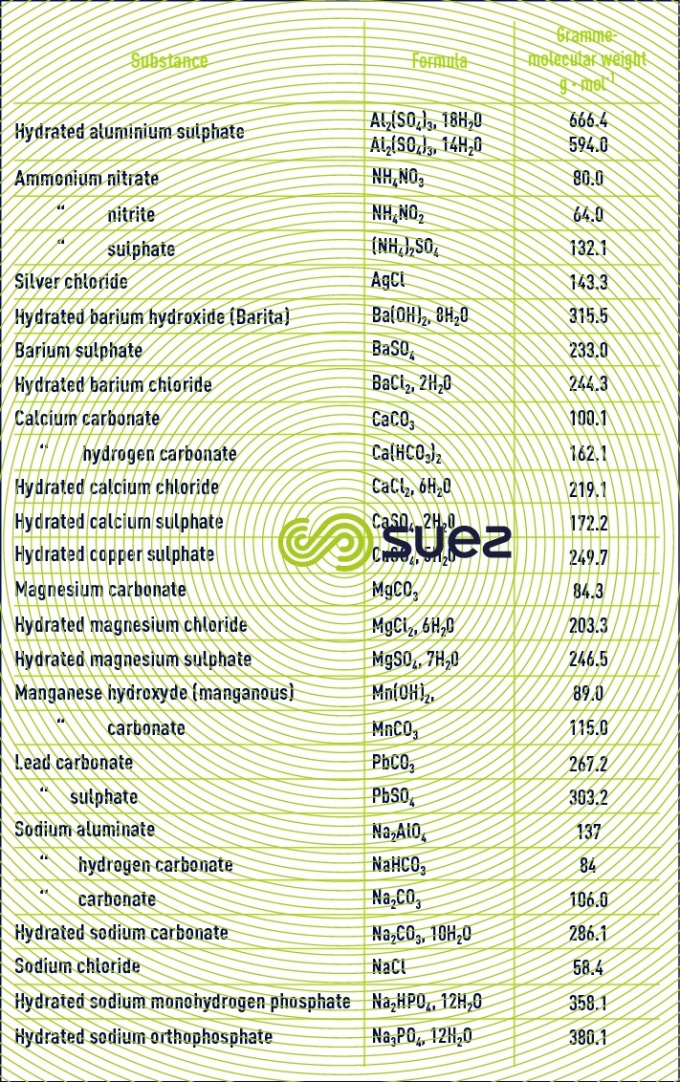
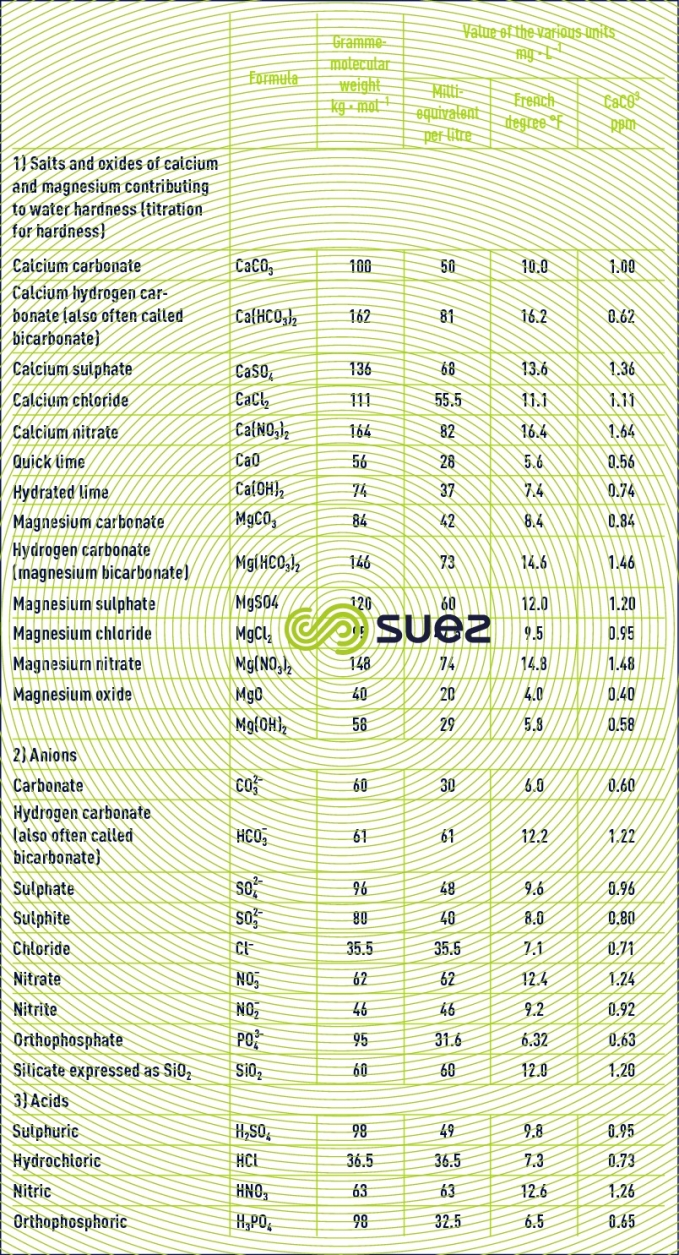
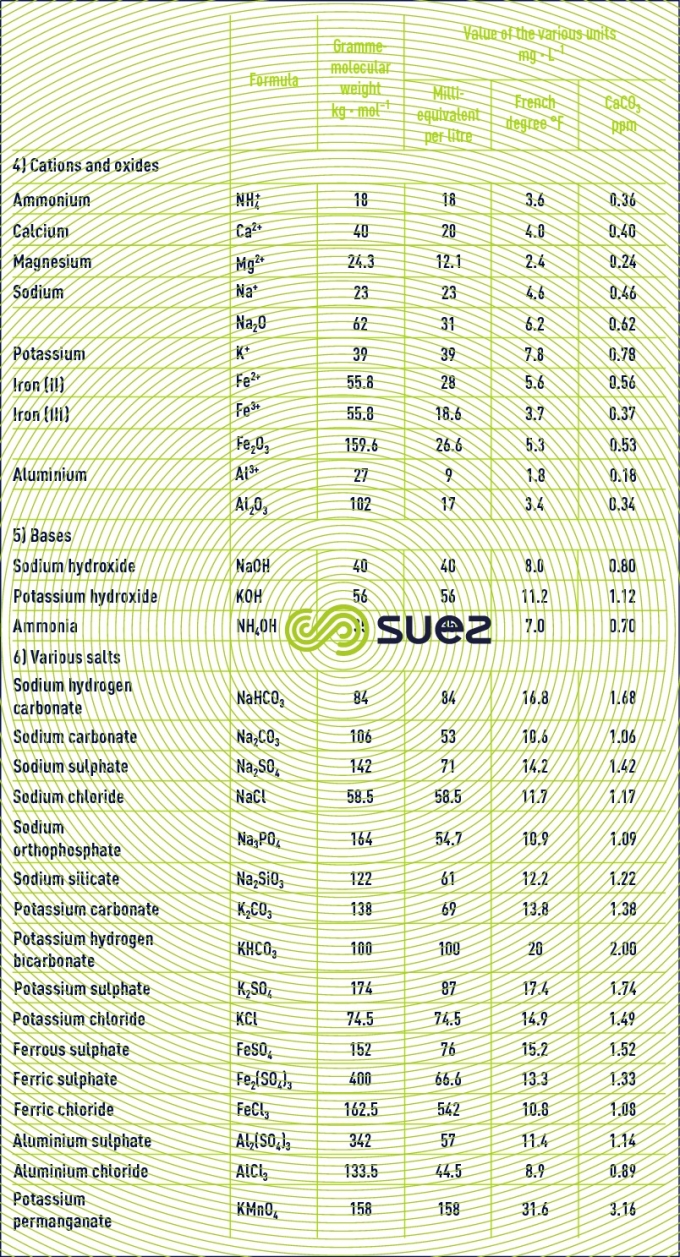
Table 22 provides the formula and gramme-molecular weight for the main salts used in water treatment.
For the organic substances, we often used the so-called semi-developed formula that indicates the presence of certain functional groupings within the molecule. Consequently, acetic acid is written CH3COOH to show that there is a CH3 group bonded by one carbon atom to the carboxylic group COOH.


rules applicable to the compounds naming and to the formulae writing
Organic compounds are named according to the nomenclature of the International Union of Pure and Applied Chemistry (IUPAC). However, there are many customary names in daily use.
Any research carried out in various chemical catalogues or dictionaries must start out with the empirical formula.
The French Chemistry Society published comprehensive rules on the Inorganic Chemistry Nomenclature (February 1975). We quote below a few fundamental paragraphs :
- In formulae, the electropositive component (cation) must always be placed first, e.g. KCℓ, CaSO4 . When the compound contains more than one electropositive or electronegative constituent, the sequence within each class should follow the alphabetical order of the symbols. Acids are treated in the same way as hydrogen salts; e.g. H2SO4 and H2PtCℓ6; please refer to letter combinations for the hydrogen position.
- in the case of binary compounds of non-metallic elements, the leading component will be the one that is shown first in the following list: B, Si, C, Sb, As, P, N, H, Te, Se, S, At, I, Br, Cℓ, O, F. E.g. : NH3, H2S, SO2, C,O2, OF2.
- salts containing acid hydrogen atoms. The names of these salts are formed by adding the prefix ‘hydrogen’ to the name of the anion to indicate the presence in this salt of the replaceable hydrogen. Clearly, this type of salt cannot be termed an acid salt.
Examples :- NaHCO3: sodium hydrogen carbonate;
- LiH2PO4: lithium dihydrogen phosphate;
- KHS: potassium hydrogen sulphide.
- single salts, triple salts.
- cations: in formulae, all cations must come before the anions.
- with the exception of hydrogen, cations must be cited in alphabetical order; this order may be different in formulae and in names. E.g. KNaCO3: potassium sodium carbonate.
- acid hydrogen: when rule 6.2 does not apply, hydrogen is written as the last of the cations. E.g. NaNH4HPO4, 4H20: ammonium hydrogen phosphate and sodium with 4 molecules of water.
- anions: anions must be quoted in alphabetical order; this order may be different in formulae and in names.
concentration evaluations
using the milliequivalent
In order to facilitate calculations, it has become the norm to evaluate analysis results not in grams per litre but in gram-equivalents per litre. The sub-multiple is the milliequivalent per litre (meq · L–1).
For instance, the gramme-molecular weight of chlorine being 35.5 g, if water contains 2 g of chloride per litre, this result can be expressed by writing that it contains :

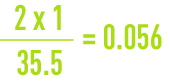
gramme-equivalent of chlorine per litre (56 meq · L–1).
In the case of a polyvalent element, the value of the concentration unit "meq · L–1" unit of concentration, expressed in mg · L–1, is the gramme-molecular weight divided by the valency. For instance, in the case of calcium, a bivalent element having a gramme-molecular weight of 40 g, a 1 meq · L–1 concentration corresponds to :

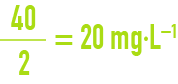
This notation has the advantage of allowing saline concentrations to be calculated directly. If, using the above example of water containing 56 milliequivalents of chloride per litre, we have a pure solution of calcium chloride, then the CaCℓ2 concentration will be :

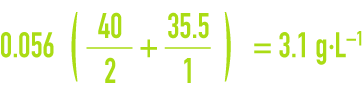
and the corresponding calcium concentration will be :

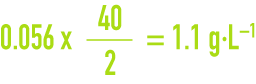
We often need to know not the detail of the different dissolved salts but the anion and cation balance. The equivalent notation system will immediately provide us an idea of this balance.
using degrees (table 23)
The concept of equivalent and milliequivalent has the advantage of being international. However, in France, the degree (1 °F = 0.2 milliequivalent per litre) is frequently used as is the ppm of CaCO3 (1 ppm CaCO3 = 0.02 milliequivalent per litre) in England and the USA, or the German degree (1°dh = 1.786 °F or 0.3572 milliequivalent per litre).




Bookmark tool
Click on the bookmark tool, highlight the last read paragraph to continue your reading later












