heavy metals removal
Reading time:behaviour within the systems
Heavy metals are extremely diverse and their removal at the different stages in a treatment system will vary from element to element:
coagulation-flocculation-settling action
Coagulation using aluminium and iron salts is very effective for removing silver, chromium (III) and tin; lead, vanadium and mercury levels are reduced by between 50 and 90%; copper (in some cases), cadmium, zinc, nickel and barium are not effectively removed. Cobalt, molybdenum and chromium (VI) are not reduced at all. Chromium (VI) can be satisfactorily removed by reducing it to chromium (III) with ferrous sulphate, precipitating hydroxides.
The amounts of PAC normally used (20 g · m–3) have little effect on the elimination of heavy metals.
action of sand filtration
Having no inherent action, filtration merely removes the metals contained within the floc that has escaped from the settling tank.
action of filtration through GAC
During a second stage, filtration through GAC will result in a satisfactory reduction of unwanted or toxic ions. Silver and mercury will be totally removed while lead and copper levels (excluding nickel) will fall below the guidelines set.
action of prechlorination
Combined with coagulation-sedimentation, sand filtration and filtration through GAC, chlorination will improve heavy metal removal, especially when the amount of chlorine used is slightly higher than that applicable to the critical point.
action of carbonate removal
Carbonate removal using lime, albeit partial, will be accompanied by a noticeable reduction in most heavy metals (except Cr(VI) and organic Hg).
special case of the european standards
Table 11 summarises the effectiveness of the main processes used to eliminate metals covered by the French decree 2001/1220.

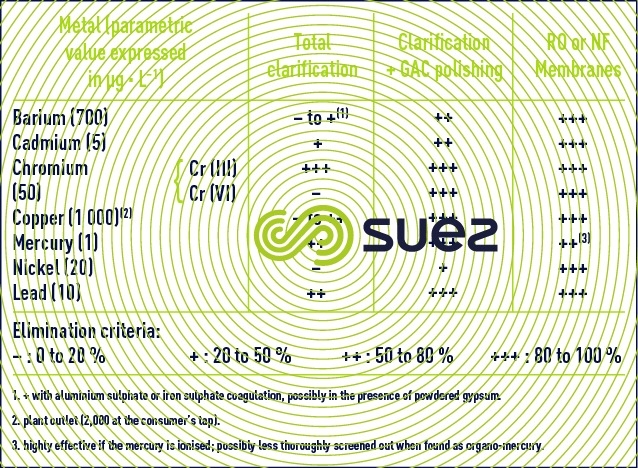

Nickel removal can be improved during clarification after pre-chlorination in the presence of PAC. One of the following treatments can be used to remove the three most difficult metals (Ba, Cd and Ni) to eliminate by conventional systems:
- carbonate removal using lime ("catalytic" at pH 9.5 or conventional at pH 10-11);
- cation exchanger resin.
the lead problem
It was just noted that lead is not difficult to remove but that, in fact, it is rarely found in raw water: it is most frequently caused by the dissolution (corrosion, see lead.) that occurs during distribution, when the mains system still has lead pipes or lead welds (brazing) (primarily: branch pipes and domestic systems). The standard applied is progressive :
- up to the 25th December 2013: 25 mg · L–1; this limit can often be met if the water undergoes adequate treatment;
- film-forming product (designed to deposit a protective layer of lead hydroxyphosphate); phosphoric acid, sodium or zinc orthophosphate and/or polyphosphate;
- remineralisation-neutralisation of extremely soft and acid water;
- carbonate removal-partial softening using lime or sodium hydroxide on water with a high calcium alkalinity;
- nanofiltration;
- after the 25th December 2013: 10 mg · L–1; after that date, no mains distribution system will be able to include components made of or containing lead such as some materials like galvanised steel, brass, etc. Other than replacement pure and simple, there are alternative solutions: retubing (e.g. polyethylene), lining (epoxy, latex)...
Bookmark tool
Click on the bookmark tool, highlight the last read paragraph to continue your reading later












