eliminating ammonium
Reading time:Physical-chemical or biological processes can be used to remove the ammonium present in water.
physical-chemical processes
As previously reviewed (see section the oxidants and disinfectants), ammonia removal via reaction with chlorine is possible when the amount of chlorine dosed rises above the critical point. However, disinfection by-products (organo-chlorines, THM etc.) often appear under these conditions and their presence is unwanted (see section pollution generated by water treatment). Therefore, this technique only applies when the water contains lowamounts of precursors. ie.
- water having low OM content;
- at the end of a treatment train, on clarified and polished water. This applies in particular when making up the shortfall in biological treatment used to remove ammonia (cold water seasons, for instance). Accordingly, the Morsang-sur-Seine (France) plant (figure 9 in polishing: removal of organic matter and micro-pollutants) is equipped with a plug-flow,polished water tank at the end of the line.
The other oxidants (ozone, CℓO2, chloramines, KMnO4) are ineffective for removing ammonia.
Ion exchange: clinoptilolite (natural zeolith) has occasionally been suggested for removing NH4 but is expensive to use.
biological removal of ammonia (nitrification)
utilisation conditions
Please refer to section nitrogen cycle, suspended growth (activated sludge) process and nitrogen conversion for the conditions applicable to the development of bacteria responsible for biologically oxidising ammonia to nitrite and then to nitrate.
As NH4 levels in raw water are quite low, in principle, nitrification during drinking water production takes place through filters (attached growth) that are either dedicated by selecting granular media (biolite) that encourages bacterial attachment, or through sand and/or GAC filters that may be required for treatment on other grounds.
Further requirements include:
- sufficient amounts of oxygen;
- addition (or presence) of phosphorous to encourage bacterial growth;
- sufficient concentration of hydrogen carbonates (TAC): bacteria are autotrophic and take the carbon required for their growth from hydrogen carbonates;
- an appropriate pH (> 7.5);
- a sufficiently high temperature: below 8 to 10°C, bacterial metabolism declines rapidly and ammonia oxidation slows considerably (and produces nitrites) or may even be totally inhibited below 4 to 5°C.
- total absence of any residual disinfectant.
Additionally, one needs to allow for a 1 to 3 months natural seeding period to ensure that the process becomes fully effective.
Utilisation techniques differ mainly through the support medium used for bacterial attachment, the flow direction of the water to be treated and the presence or absence of air that is continuously injected into the nitrification reactor.
It should be noted that in drinking water treatment, reasoning tends to be based on the ammonium ion NH4+ and not, as in the case of wastewater, on N-NH4+ = nitrogen in the ammonium.

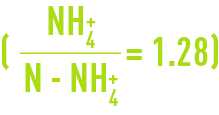
pozzolana filters
Historically, this is the first technique applied but it is subject to onerous operating restrictions. In fact:
- Pozzolana (> 1 cm) cannot be backwashed, even using air and water; therefore,the filter has to be shut down at regular intervals and soaked in chlorinated water;
- every 2-3 years, this material has to be removed from the filters and replaced.
Therefore, this technique was dropped and superceded by the Nitrazur N.
biolite filters
In section nitrogen conversion the effect of temperature on nitrification kinetics was reviewed and this aspect was reminded.
Additionally, it is known that the biological oxidation of ammonia requires an amount of oxygen equal to:


a) When tthe amount of oxygen required for nitrification is likely to be contained within the water to be treated, then the reactor does not need aerating. In practice:
- when NH4+ < 1 mg · L–1, only one conventional sand filter needs to be used providing that the water is properly pre-aerated;
4+
–1
nitrifying filter bed
LFN in french) loaded with biolite is included; oxygen is injected into the water during a preliminary aeration step, either by cascade or by dissolution using porous diffusers. That applies to the “Eau & Force” plant at Mont Valérien, France
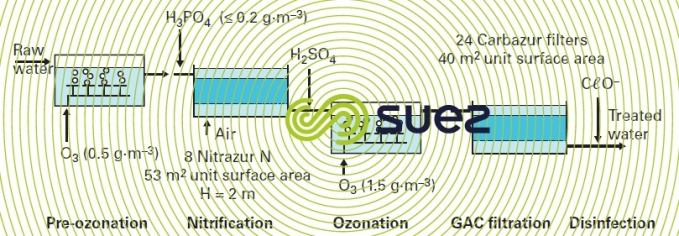
polishing: removal of organic matter and micro-pollutants
- an aeration cascade;
- pre-ozonation;
- clarification using the Densadeg;
- filtration/nitrification using an nitrifying filter bed:
- biolite 1.1 mm;
- depth: 1 m;
- velocity ~ 7 m · h–1;
- ozonation;
- filtration through GAC (Biflux filters).
b) When the NH4+ concentration is such that there is not enough oxygen dissolved in the water, one needs to utilise an aerated reactor; such as the Nitrazur N (see section nitrogen conversion and figure 43) of which there are two variants :
- counter current reactors where water flows from the top downwards and air from the bottom upwards (e.g. the Louveciennes (France) DWTP figure 36 and photo 23);
- co-current reactors where both water and air flow from the bottom upwards (e.g. the Okhla plant in India).
These reactors operate at a velocity of approximately 10 to 15 m · h–1 and use a 0.3 to 1 air/water volume ratio.





The choice of one or other type will be guided by the amounts of NH4+ that has to be nitrified and, therefore, the oxygen requirements; in effect, an air flow rate that is high compared to the water flow rate can create binding phenomena in a downflow reactor; on the other hand, with an up-flow (co-current) reactor, additional filtration will be required because low turbidity levels cannot be guaranteed with this type of reactor.
filters using floating filtration media
The Filtrazur (see section filtrazur filters) is an attractive alternative to the foregoing nitrification reactors.
Use of a material that is lighter than water also allows filtration to take place from the bottom upwards and, if necessary, for air to flow upwards.
In fact, non-aerated Filtrazur are used with 1 to 1.5 mg · L–1 concentrations of NH4+ (Aubergenville) and aerated versions for higher concentrations (Jang-Xou, China).
biological treatment of water containing ammonia, iron and/or manganese
When these three elements are present, the treatment sequence in the stability diagram can be seen in figure 37. It follows that iron can be eliminated biologically without nitrification, but not manganese.

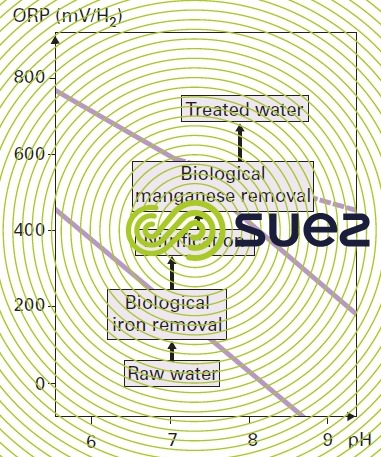

Additionally, the operating conditions applicable to biological iron removal (high velocity, short contact time, dissolved O2 limitations) will be in opposition to those required for nitrification; therefore, biological iron removal will often have to be carried out at a later stage, calculated on the basis of the NH4+ that has to be removed and on the temperature of the water; biological manganese removal can then be carried out in the same reactor provided nitrification is complete before the treated water is discharged.
Depending on the respective concentration levels of the three elements and on the other properties of the water, many combinations will be feasible for example:
- low Fe, Mn, NH4+ concentrations: physical-chemical iron removal, nitrification and biological manganese removal in the same filter;
- average or high Fe content and low Mn, NH4+ ≤ 1.5 mg · L–1 : biological iron removal followed by intensive aeration and filtration through sand or nitrifying filter bed depending on the water’s precise NH4+ concentration and temperature;
4+
–1
- biological iron removal;
- nitrification using the Nitrazur N;
- polishing filtration where nitrification and manganese removal will be completed simultaneously using the biological process.
Note: for surface waters containing suspended solids where drinking water production demands a comprehensive clarification treatment including polishing, nitrification will be seen to occur during several of the stages applied. E.g. raw water storage, clarifier sludge beds, sand filters, GAC filters.
Bookmark tool
Click on the bookmark tool, highlight the last read paragraph to continue your reading later













