carbonate removal and/or softening
Reading time:Sections removing hardness (calcium and magnesium) and classic schemes used describe the processes used to correct water that is too hard by using the chemical precipitation (carbonate removal) or ion exchange methods, respectively.
softening through carbonate removal
When the water has a high TH accompanied by a significant M-alk., the water can be softened by using lime to remove the carbonate.
This carbonate removal can be carried out:
- "catalytically" by Gyrazur when no concomitant clarification is needed and when the magnesium content is low;
- by clarification in a Densadeg or any other sludge recirculation equipment; ferric chloride will then have to be used as the clarification reagent;
- or by separating the clarification and carbonate removal functions when the carbonate removal inhibitors are present from time to time (refer to the Hanningfield (UK) example provided further on).
For drinking water treatment, it will be necessary to:
- either decarbonate completely only part of the throughput and mix it with the remainder that may have to be clarified if necessary;
- or partially decarbonate the water as it is being clarified. This is the solution that has to be adopted when iron and manganese also have to be removed.
When the raw water contains more calcium than bicarbonate (CaH >> M-alk.), carbonate removal using sodium hydroxide may be an option (alternative: lime + sodium carbonate).
In order to ensure that the water is pleasant to drink, a certain M-alk. will have to be re-instated by mixing the water with a fraction of water from which the carbonates have not been removed.
Both of the following examples illustrate the range of systems that can be used to treat surface water.
The Basse Vigneulles (France) plant uses a Densadeg followed by simple sand filtration using material with a homogenous particle size (suspended solids concentration at a Densadeg outlet are always well below 10 mg · L–1).
The Hanningfield (UK) plant (230,000 m3·d–1) processes reservoir water that, in addition to significant levels of micro algae, organic matter and pesticides, etc., is too hard (24 °F to 42 °F).
The treatment has been designed on the basis of the following sequence: clarification – carbonate removal (lime) – filtration – polishing (O3 + GAC).
Previously, Accelators had been used for carbonate removal. When the plant was extended, the Gyrazur process (see the gyrazur, a granular contact mass reactor) was selected for partial water softening. Figure 47 summarises the treatment system that comprises :

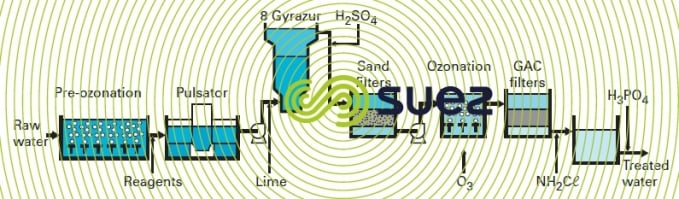

- pre-ozonation (0.5 to 2 g · m–3);
- addition of coagulant (5 to 22 g FeCℓ3·m–3) and flocculant (0.05 to 0.2 g polymer · m–3);
- clarification in three Pulsators, each having a 1,118 m² surface area; in particular, organic substances potentially inhibiting carbonate removal are eliminated;
- lime injection (80 to 130 g · m–3);
- "catalytic" carbonate removal in eight Gyrazur (see section photo 26 the gyrazur, a granular contact mass reactor) ;
- H2SO4 (0 to 20 g · m–3) injected to neutralise residual free alkalinity;
- filtration (sixteen dual media filters and eight Aquazur V);
- oxidation using the Perozone process (0 to 1 g H2O2·m–3 and 2 to 4 g O3·m–3);
- filtration through GAC (twelve Carbazur GH);
- chlorine (as chloramine) and H3PO4 (to limit lead dissolution in the mains system) addition.
Solutions will also be tailored to each case for groundwater. E.g.:
- at Isle-Adam (France), a Densadeg operating for carbonate removal was constructed upstream from an existing pressurised biological iron removal unit;



- at Villeneuve-la-Garenne (France), the plant already included nitrification followed by sand filtration; the plant was then refurbished by adding partial water softening with Gyrazur, after injecting lime slurry, and completing the treatment by adding a small amount of FeCℓ 3, dual media filtration, pH correction using H2SO4, ozonation and final chlorination for mains protection purposes ;
- at Ratingen (Germany), on the other hand, (see Iron removal and figure 27), the Gyrazur was positioned at the inlet for iron and manganese removal coupled with partial carbonate removal; the water is then aerated, before being clarified and nitrified over dual media filters.
softening over resin
It is absolutely essential that the regulations applicable in the country concerned are checked in order to find out which resins can be used for this purpose.
Cationic resins exchange their Na+ ions for the Ca2+ and Mg2+ ions in the water. The anions are not modified.
Water obtained in this way has a zero TH: it is corrosive and unpleasant to the palate; a certain residual TH needs to be maintained (8 to 15°F) by only softening part of the output which is then mixed into the remaining output.
The advantage of this type of softening is that it does not generate solid waste and that it can be carried out under pressure.
Carboxylic resins are used to fix calcium ions bound to bicarbonates; the water produced will be acidic because the bicarbonates are converted into CO2; consequently, the treatment has to be supplemented by the elimination of CO2 through stripping, and then by a final neutralisation using sodium hydroxide.
electro-carbonate removal
When the objective is only a minor reduction in calcium, precipitation can also be undertaken by generating OH– ions on the surface of an electrode during the water electrolysis process. This is electro-carbonate removal and it is generally carried out in-line.
Bookmark tool
Click on the bookmark tool, highlight the last read paragraph to continue your reading later

















