organic matter removal
Reading time:Some groundwater, for instance, may only pose problems concerning OM removal. This type of problem can also predominate in surface water. The applicable treatment may vary widely depending on the nature of this OM.
nom – colour
Humic and fulvic acids (a proportion of which is responsible for coloration) can be fairly effectively removed by flocculation-coagulation; however, the coagulant demand may be high; as a result, direct filtration can only eliminate 10 to 30%; on the other hand a 50 to 80% reduction is achievable in a complete clarification system with optimised coagulation and, if necessary, with PAC used in the settling tank; under these optimum conditions, the molecules responsible for coloration will usually be completely removed; in some cases, stringent requirements will impose an O3 or GAC polishing treatment, especially when residual coloration persists and/or too many THM precursors and other oxidation by-products remain after clarification (see section polishing: removal of organic matter and micro-pollutants).
taste and odours
With the exception of taste linked to water mineralisation, the chlorophenol taste induced by poorly applied chlorination and the odour caused by the residual chlorine, unpleasant taste and odours are usually caused either by industrial discharges or by the metabolism of algae, actinomycetes and bacteria (see section micro-organisms for which freshwater is their natural habitat) ; these nuisances must be identified in all cases: establishing their source, the frequency with which they occur, their nature (tasting and smelling, CGMS analysis whenever possible) in order to establish the possibility for treatment. Treatment action can be summarised as follows :
aeration
Aeration is used to remove unpleasant odours caused by the presence of H2S or of certain volatile organic compounds (toluene, ethylbenzene).
clarification
Clarification has no appreciable effect but can, however, create unpleasant odours (dead zones, anaerobiosis when the system is shut down) in the absence of optimised hydraulics and operation.
oxidation
Ozone is the most powerful oxidant for this purpose but is only effective on some compounds (e.g. phenols, avoiding the subsequent formation of chlorophenols when chlorination is carried out; some algae metabolites, etc.); poorly managed ozonation can also create characteristic tastes and odours (flowers, vegetables, etc.).
activated carbon
Activated carbon can be used in powder form, when bad tastes appear intermittently, provided they can be detected as they enter the raw water. As soon as the amount of PAC exceeds an average of 15-20 g · m–3 over a sufficient period, filtration through GAC becomes a more cost-effective option, unless the plant includes clarification that can be adapted to the "extended Cristal" process (see section membrane processes).
combined ozone-activated carbon treatment
This is the treatment of choice for eliminating tastes and odours. It can also reduce the chlorine demand in the network and, therefore, the final amount injected. It is being used in an increasing number of plants.
Additional information, specifically on algae metabolites, has been provided in problems associated with algae and zooplankton.
organic micro-pollutants
As clarification is ineffective on most of these (apart from detergents that it can reduce by up to 50%), the determining treatment will be polishing (polishing: removal of organic matter and micro-pollutants) : PAC, GAC, O3 + GAC, Cristal, extended Cristal… Further details can be found in sections polishing: removal of organic matter and micro-pollutants, membrane processes and membrane processes.
special case of chlorinated solvents
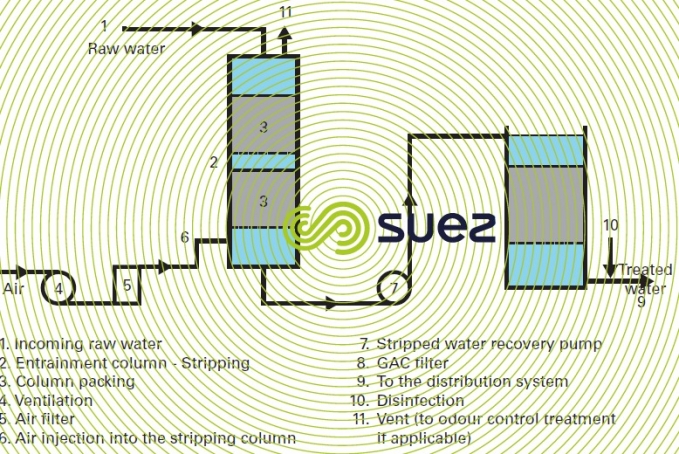
When a water’s organic contamination is caused solely by large quantities of volatile compounds (e.g. trichlorethylene, tetrachlorethylene, chloroform, carbon tetrachloride…), elimination by air stripping is an option. This applies to some aquifers. After stripping, polishing can be carried out by filtration through activated carbon as indicated in figure 42.


Bookmark tool
Click on the bookmark tool, highlight the last read paragraph to continue your reading later












