main adsorbents
Reading time:activated carbon
Activated carbon is produced as the result of heat treatment that is carried out in a stringently controlled environment: the thermal activation (drying, carbonisation at 500-600 °C and controlled oxidation at 850-1 000 °C) of various natural materials (coal, lignite, wood …).
This treatment only leaves behind the carbonaceous skeleton of the materials and the departure of volatile products leaves an entire network of pores (figure 71), explaining the extensive developed surface areas. However, pore size and structure will vary depending on the source of the carbon and on the activation treatment applied, explaining the difference in properties of the activated carbons obtained (capacity, friability); it should be noted that the friability problem can be resolved by producing the carbons as reconstituted granules (e.g. extruded as cylinders measuring a few mm).

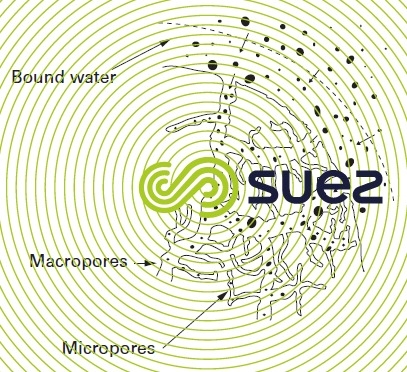

Experience has demonstrated that the “right” activated carbons are very wide spec- trum adsorbents: most organic molecules will attach to the activated carbon surface; the most badly retained molecules will be the most polar molecules as well as very low gramme-molecular weight linear molecules (simple alcohols, primary organic acids…). Low polarity molecules that emit taste and odours, relatively high gramme-molecular weight molecules are, for various reasons, well adsorbed over carbon.
In addition to these adsorbant properties, activated carbon also constitutes an excellent bacteria support capable of degrading the biodegradable fraction of the adsorbed phase (see the BAC (biological activated carbon concept)). This enables part of the support to undergo biological regeneration.
Activated carbon also enjoys reducing properties with regard to oxidants.
main applications
Activated carbon is used:
- in drinking water or high purity industrial water finishing treatments; in these applications, activated carbon fixes the dissolved organic compounds that have evaded natural biological degradation (water way self-purification) and also upstream physical-chemical treatments. Indeed, initially, activated carbon had been used to improve the organoleptic qualities of a water by removing the organic matter responsible for taste, odour and colour. As pollution increased, their use became extended to the removal of many pollutants and micro-pollutants such as phenols, hydrocarbons, pesticides, detergents and even some heavy metals that were not totally removed through clarification. Activated carbon was also used to remove THM precursors and disinfection by-products;
- in industrial wastewater treatment, when the effluent is not biodegradable or when it contains organic toxic elements that prohibit the use of biological techniques. In this case, activated carbon can be used to selectively screen out toxic elements and, subsequently, produce an effluent that is normally biodegradable;
- in “tertiary” treatment applied to industrial water or wastewater. Carbon fixes dissolved organic compounds that do not take to upstream biological treatment and can thus remove a greater or lesser proportion of residual COD ("hard" COD);
- for reducing oxidants
One of the properties of activated carbon is its reducing action on oxidants (chlorine, chlorine dioxide, permanganate, chloramines, ozone…).
Water that has undergone an excess chlorination treatment will be dechlorinated according to one of the following reactions :

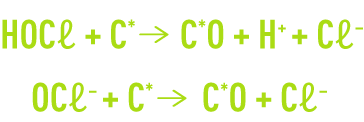
Where C* and C*O represent respectively the unadulterated surface and the oxidized surface of activated carbon. These reactions can be continued by forming CO2, generating a certain carbon consumption that, therefore, sometimes requires compensating in the form of a partial reload.
The half-chlorination length characterises this dechlorination action: this is the filter bed depth that, for a given velocity, causes the chlorine concentration in the water to fall by half (see analysis of the adsorbent capacity of a carbon). pH will have an impact on this length.
In practice and depending on the temperature, we use the available chlorine content and the permissible residual chlorine margin in bed loads ranging from 5 to 15 volumes of water to each volume of activated carbon and per hour.
This reducing action also applies to chloramines that are broken down into nitrogen and hydrochloric acid. However, the kinetics are slower than in the case of available chlorine (much greater half-dechlorination length); thus, we need to significantly reduce the bed loads if we want to obtain comparable results.
This action is applied for eliminating, where necessary, reagent residues: ozone (odour, corrosion), permanganate (coloration).
Anything that is likely to interfere with the contact between the carbon and the water to be treated will also affect carbon’s dechlorination capacity: calcium carbonate deposits, surface saturation through the adsorption of various pollutants….
Conversely, this oxidation of the carbon surface shows up as reduced adsorption efficiency with regard to the less easily adsorbable pollutants.
other adsorbents
In addition to the natural adsorbents already quoted, new adsorbents have been developed:
- mineral adsorbents: various aluminas and metal oxides; although some have large specific surface areas (300 to 400 m2 ·g–1), these solids adsorb more selectively than carbon. Their capacity is closely linked to pH and to their mesoporosity. Below the iso-electric point, only the negatively charged molecules will be adsorbed on positive sites. At their current state of development, these adsorbents cannot compete with activated carbon for adsorbing OM found in water. However, some of these solids, such as the aluminas or the ferric oxyhydroxides, even the latter charged with manganese oxide, are of great interest with regard to the removal of arsenic, fluorine and phosphates;
- organic adsorbents: macromolecular resins having a specific surface area ranging from 300 to 750 m2 ·g–1; compared with activated carbon, they have a fairly poor capacity; however, these resins have better adsorption kinetics (utilising between 5 and 10 vol · vol–1 · h–1) and are often more easily regenerated (low bond energy).
We should also mention “scavengers”, anionic macroporous resins (see Adsordant resins and special resins). However, these resins have a smaller specific surface area and owesome of their activity with regard to polar substances (such as: humic acids, anionic detergents) to their ionic charge, thus standing out from other adsorbents
Bookmark tool
Click on the bookmark tool, highlight the last read paragraph to continue your reading later













