reducing agents
Reading time:Treatments using physical-chemical reduction meet a number of very specific objectives such as:
- removing oxygen;
- reducing hexavalent chromium;
- reducing nitrites;
- destroying residual oxidants.
chemical reduction of oxygen
We use sodium sulphite or ammonium bisulphite which, although more expensive, is easier to use and has a much more extensive buffer effect.



Three applications are widely encountered: boiler water treatment, conditioning cooling closed circuits and conditioning secondary recovery water (oil fields).
reduction of hexavalent chromium
Chromium is mainly present in IWW as a trivalent or hexavalent. Chemical removal of total chromium takes place in two stages: hexavalent chromium is reduced into trivalent chromium that precipitates as a hydroxide. The usual forms of hexavalent chromium: chromate ion CrO42–, dichromate ion Cr2O72– and chromic acid H2CrO4.
Table 42 lists the different reduction methods together with the theoretical amounts of pure reagents involved. The most widespread technique involves sodium bisulphite.
reduction of normal oxidising agents
The residual oxidising agent must, in some cases, be reduced or totally eliminated:
- when preparing drinking water or after UWW disinfection in order to limit the subsequent formation of unwanted by-products;
- before processing the water through a membrane or ion exchanger resin;
- after transporting drinking water over long distances;
- before discharging or recycling gas from ozone utilisation applications.



elimination residual chlorine
The most frequently used reducing agents are sulphur dioxide (gas supplied in its liquefied form) and sodium bisulphite (available as a 23-24% SO2 aqueous solution) that act according to the following reactions:

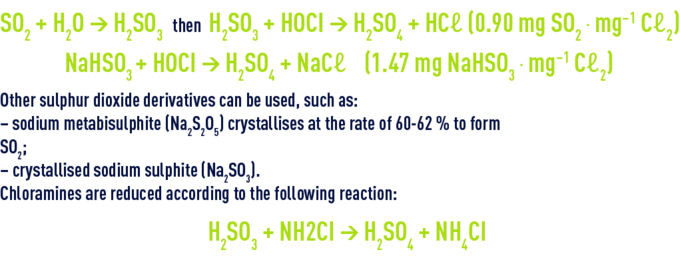
The reactions take place quickly in such a way that, in drinking water distribution systems, the contact time available in the pipelines will suffice. The amount of reducing agent dispensed is governed by the residual chlorine content.
Upstream from reverse osmosis, a slight excess of reagent is being used at present (approximately 20%) in relation to stoichiometry.
Note: Sodium thiosulphate must not be used upstream from reverse osmosis because there is a risk that mineral sulphur will form (resulting in the modules becoming fouled) through a thiosulphate decomposition secondary reaction (dismutation).
In the case of boiler water, chapter treatment and conditioning of industrial water describes the physical and chemical treatment conditions (nature of reagents) used. In agri-food industries, pure anhydrous sulphite (or ascorbic acid under exception conditions) is normally used.
Activated carbon can be used in dechlorination applications. Contact time in the columns tends to extend to a few minutes (see section main adsorbents). This technique is mainly used in the beverage industry or upstream from demineralisation lines. It is not recommended upstream from reverse osmosis: danger of bacteria proliferation, discharge of fines.
eliminating residual ozone
Three techniques can be used to break down the residual ozone found in gas at the outlet of an ozonation reactor :
- thermal destruction by heating to 300-350°C for 2 to 4 seconds;
- destruction by incinerating the activated carbon.
The 2 O3 + 3C → 3CO2 reaction involves the consumption of 0.38 g of activated carbon for every g of ozone destroyed. This process is usually carried out on an activated carbon bed heated to 60 - 80°C in order to ensure a high level of performance. During the reaction, the activated carbon particles are reduced to a powder; the danger of explosions appears but is kept under control by humidification;
- catalytic destruction.
There are many catalysts that are suitable for breaking down ozone. The most conventional formulations concern manganese dioxide and palladium deposited on alumina. Catalysts operate at temperatures of between 50 and 70°C in order to avoid inactivation caused by the condensation of water contained in the gas to be processed.
There are alternative techniques but these require more extended contact times, thus restricting their application:
- photochemical destruction by UV irradiation at a 254 nm wavelength for a few minutes;
- chemical breakdown in a scrubbing tower using ferrous sulphate, sodium chlorite, hydrogen peroxide…
Based on the same principles, when the natural breakdown of liquid phase residual ozone does not take place sufficiently fast, various agents can be used :
- activated carbon;
- anthracite;
- UV;
- bisulphite;
- iodide or bromide that produce free iodine or bromine capable of acting as a disinfectant, especially in swimming pool water applications.
Bookmark tool
Click on the bookmark tool, highlight the last read paragraph to continue your reading later












