demineralisation line calculation principle
Reading time:The following data will be required for calculation purposes:
- raw water total alkalinity [M-alk.] in °f; °f = french degree
- raw water strong acids salts [SSA] in °F (SO4 + Cℓ + NO3);
- silica content [SiO2] (1° f= 12 mgl–1 SiO2);
- the water’s carbonic acid content and, possibly, after the carbonic acid has been removed, TCO2;
- volume of water V to be provided between regenerations, in m³, including process water if applicable;
- hourly output Q in m³/h;
- resin exchange capacity C expressed in French degrees per litre of compacted resin. (Degrees can be replaced by milliequivalents, where one milliequivalent = 5°F).
For rough calculation purposes, the exchange capacity is obtained from documents for each resin or from calculation software supplied by the manufacturer. First calculate the anion exchanger having a capacity C; the volume of resin to be used will be provided by the following equations:

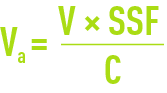
in the case of a low alkalinity exchanger and by:

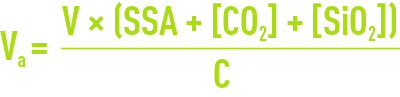
in the case of a high alkalinity exchanger.
NB: TCO2 = CO2 content entering the strong anion exchange process, i.e. the CO2 content in raw water + CO2 obtained following the dissociation of alkalinity titration via a cation exchanger, or less (1 to 1.5°f) if a CO2 removal process exists upstream to the strong basic anion exchange process.
The cation exchanger calculations are then carried out allowing for the extra water aVa required for anion exchanger rinsing. We then have:


The volumes calculated in this manner must then be compared with the hourly output to be treated. The speed of passage or the bed loading are subject to upper limits.
If Vc or Va are too low, they will have to be adjusted and this will increase the cycle volume V.
Bookmark tool
Click on the bookmark tool, highlight the last read paragraph to continue your reading later












