basic concepts on disinfection
Reading time:Disinfection consists in rendering inactive pathogenic organisms carried in water such as bacteria, viruses or parasites. Disinfection differs from sterilisation which aims at the total elimination of all germs. The germicidal action of disinfectants is based on oxidation reduction mechanisms. Thus, the effectiveness of a chemical disinfectant will be directly related to its oxidising capacity which is itself linked to temperature and pH.
The way in which a disinfectant agent works will depend on the nature of the micro-organism and on its chemical structure. As a rule:
- in the case of bacteria, the oxidant action makes the cell membrane more porous and affects nucleic acid macromolecules (DNA, RNA), inhibiting all reproduction;
- in the case of viruses, the oxidant penetrates the capsid and modifies the DNA or RNA proteins.
The importance of average germ resistance to chemical disinfection can be outlined as follows (figure 91).



Physical-chemical disinfection effectiveness is expressed as a logarithmic unit of the percentage of residual living organisms based on a prime order inactivation rate (table 24).

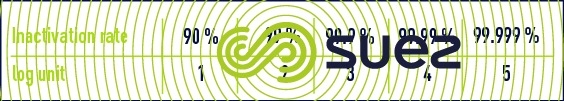

It is generally acknowledged that germ inactivation obeys a Chick-Watson type law on kinetics: N = N·exp(– k · D · T) where N and N are the germ concentrations before treatment and at treatment time T respectively, D is the amount of oxidant absorbed (concentration C for chemical agents or irradiation intensity I for UV radiation) and k is the velocity constant. Accordingly, the CT concept is used in practice to compare the effectiveness of the various chemical disinfectants.
Other phenomena also have to be taken into account when evaluation disinfection quality:
- oxidant remanence;
- regrowth of micro-organisms under the effect of repair mechanism development.
Chapter oxidation-disinfection addresses all parameters that govern disinfection effectiveness: choice of oxidising system, pathogen inactivation rate, reactor design, impact made by water quality.
Bookmark tool
Click on the bookmark tool, highlight the last read paragraph to continue your reading later












