gas dissolution (absorption)
Reading time:This operation is used to treat water (iron removal, oxidation, disinfection, biological purification) or to purify a polluted gas. Gases that have to be dissolved are usually not very soluble and the liquid film resistance to transfer will be generated in such a way that we can write :

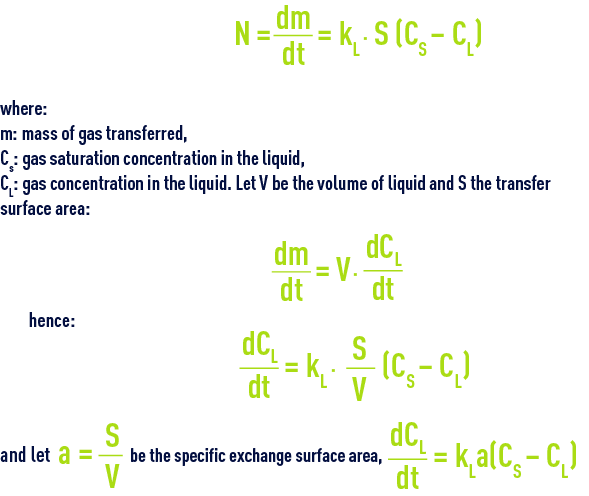
The expression KLa is termed the mass transfer coefficient whence :
a isexpressed in m–1
and KL is expressed in ms–1
KLa is expressed in s–1.
In general, in water treatment applications, absorption is accompanied by a chemical reaction that is usually a more or less fast oxidation reaction (e.g. oxidation of Fe2+, conversion of CO2 into HCO3–, disinfection, oxidation of organic matter with or without bacterial respiration …). When a gas reacts strongly with certain constituents found in water, the mass transfer coefficient will be greater than that applicable in pure water. Not only do gas solubility and diffusion capacity have to be taken into account but also the chemical kinetics that will limit the concentration in water of the O2, O3, CO2 absorbed.
There are two main types of dissolution :
- within a liquid: in order to obtain an extensive contact surface area, the gas is dispersed finely by being bubbled through the diffuser (porous component, membranes …) or by being injected through a turbine (surface mounted or submerged) or through a Venturi system (see section aeration systems) ;
- on the surface of the liquid: the contact surface is maximised by trickling the liquid through packing that may or may not be sequenced; e.g. gas scrubber, CO2 remover, CO2 dissolution …
There are also mixed solutions that combine these two dissolution modes: e.g. iron removal columns, Nitrazur N, Biofor reactors.
Bookmark tool
Click on the bookmark tool, highlight the last read paragraph to continue your reading later












